Which Terms Are Used to Identify Pure Substances
This difference is most easily seen when the temperature of a liquid is measured as it cools. Which term are used to identify pure substances.
How To Distinguish Pure Substances And Mixtures Dummies
A pure substance refers to an element or a compound that has no component of another compound or element.
. Which terms are used to identify pure substances. Substances are basically classified into two types. For example the pure sugar.
A mixture can be. It is much more difficult to break down pure. 23 Pure substances ESAZ Any material that is not a mixture is called a pure substance.
Steel Iron Gold Diamond Water Copper and many more. A phase is any part of a sample that has a uniform composition and properties. The substances that are free from any kind of mixture and contain only one kind of particle are pure.
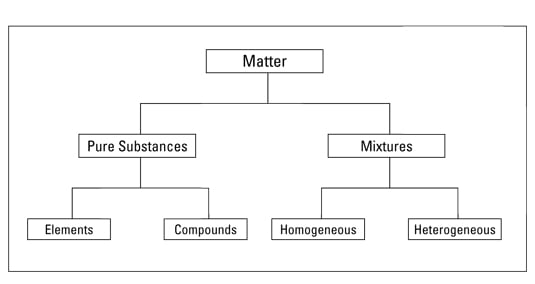
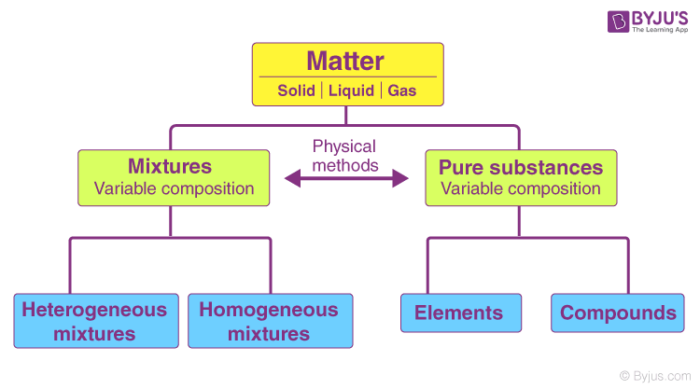
An element and a compound. Pure substances include elements and compounds. In the more general sense a pure substance is any homogeneous mixture.
Pure substances A pure substance has a definite and constant composition like salt or sugar. The term substance is fairly common and tends to be used with several different meanings in everyday language. 13 Which terms are used to identify pure substances.
Which statement is an identifying characteristic of a mixture. However in the world of physical science a substance is. Learn vocabulary terms and more with flashcards games and other study tools.
The terms element and compound are used to identify pure substances. Pure substances are made of only one type of atom or. That is it is matter that appears uniform in appearance and composition no matter how small the.
For chemistry homework the safest examples. Scientists often use filtration to. Pure substances are substances that are made up of only one kind of particles and has a fixed or constant structure.
What material is a mixture. By definition a pure substance or a homogeneous mixture consists of a single phase. Two pure substances mixed together are known as a mixture.
1 an element and a mixture 2 an element and a compound 3 a solution and a mixture 4 a solution and a compound. The material is no longer a pure substance if it has been mixed with another pure substance. Please note that air is also often considered a pure substance.
The terms element and compound are used to identify puresubstances. In chemistry a pure substance has a constant chemical composition. No matter where you sample a substance it is the same.
Over a range of temperatures. For example salt is a pure substance made only of sodium chloride. Substances are made from only one chemical or one.
A pure substance can be either an element or a compound but the composition. Start studying Identifying Pure Substances and Mixtures. A bowl of salt.
1 an element and a mixture 2 an element and a compound 3 a solution and a mixture 4 a solution and a compound. What terms are used to identify pure substances. A few examples of pure substances include the following.
Color odor density melting temperature boiling temperature and solubility are examples of physical properties. Pure substances are further classified as elements and compounds. Pure substances have a sharp melting point.
Physical properties can be used to identify a pure substance. See answer 1 Best Answer. Elements are listed on the.
While the concept of pure substances is very easy to understand reality presents a more relative idea of purity.

What Are The Types Of Pure Substances Compounds Elements Videos

What Is Pure Substance Definition Examples Difference Between Pure Substance Mixture

Mixtures And Pure Substances 4 1 4 2 And 4 3 Diagram Quizlet

Which Terms Are Used To Identify Pure Substances Brainly Com
Comments
Post a Comment